FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed
:max_bytes(150000):strip_icc()/Health-Stocksy-3418126-80bec79dd1a145419ff8a990f1b32b57.jpg)
FDA Approves Fecal Transplant Therapy for Recurrent C. Diff

Hiltzik: A judge undermines the FDA on stem cells - Los Angeles Times
T-cell and natural killer cell therapies for hematologic

One Expert's Approach in Transplant-Ineligible, Newly Diagnosed

FDA Approves 2 New Bispecifics for Relapsed or Refractory Multiple
FDA Approves Cell Therapy for Blood Cancer Patients to Cut
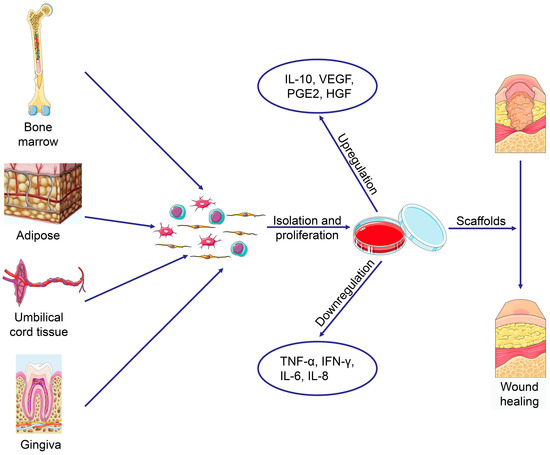
Cells, Free Full-Text

New cell therapy approaches yield fewer complications after organ
de
por adulto (o preço varia de acordo com o tamanho do grupo)