A different brand of iron tablet was analysed by Hitration with 0.0093 mol.L potassium dichromate via the
Por um escritor misterioso
Descrição
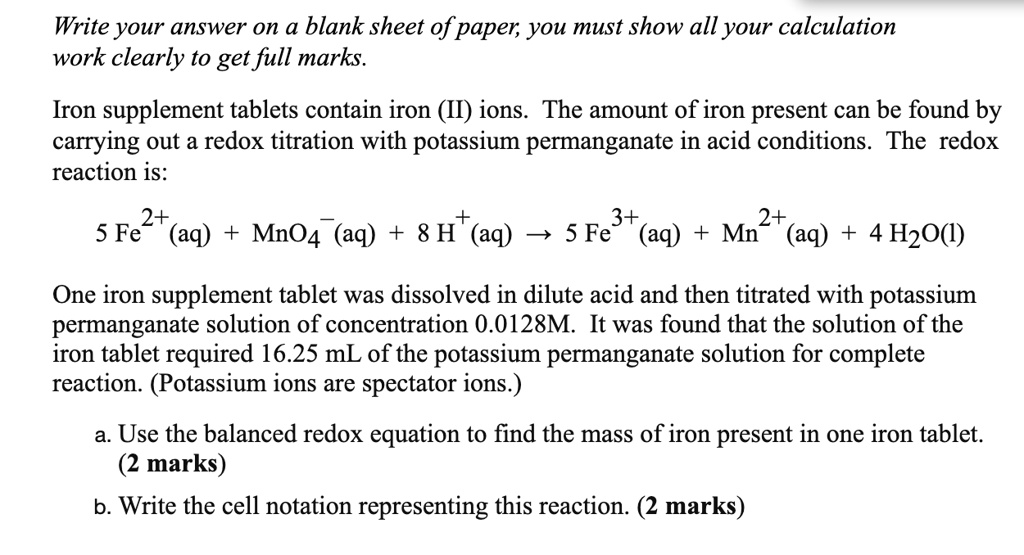
SOLVED: Iron supplement tablets contain iron (II) ions. The amount of iron present can be found by carrying out a redox titration with potassium permanganate in acid conditions. The redox reaction is

REVIEW OF FEATURES OF MERCURY CHEMISTRY OF CHIEF INTEREST TO RADIOCHEMISTS, Radiochemistry of Mercury

Free Flip-Book Chemistry Class 12th by Study Innovations. 515 Pages - Flip eBook Pages 151-200
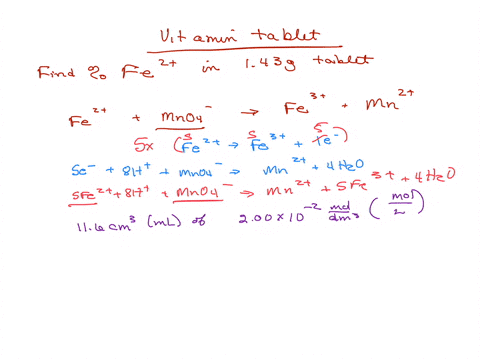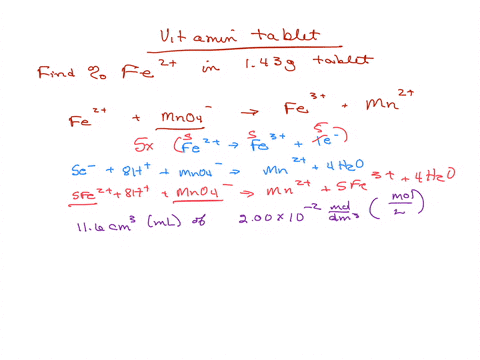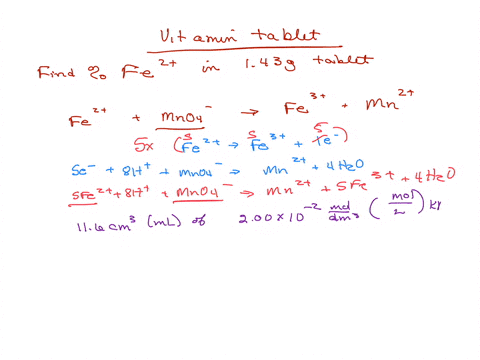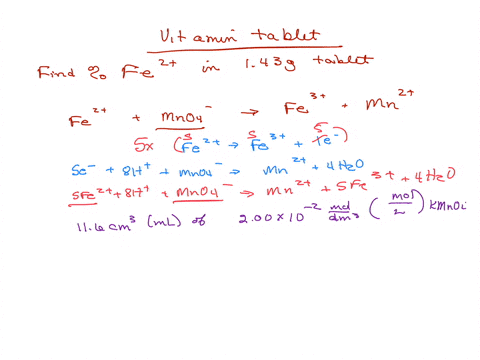
SOLVED: Iron supplement tablets contain iron (II) ions. The amount of iron present can be found by carrying out a redox titration with potassium permanganate in acid conditions. The redox reaction is

Analysis of Iron Tablet - Analysis of Iron Tablet by Shawn Silva What is the percentage of the active ingredient iron present in an iron
Solved] An 0.0152 L acidic solution of potassium dichromate is titrated

Finding the concentration of iron in iron tablets.

Analytical Chem istry - DePauw University

What amount of CuSO4.5H2O is required for the liberation of 2.54 g of I2 when it titrates with KI? - Quora

How much iron is in an iron tablet? - ppt video online download

IUPAC/CITAC Guide: Evaluation of risks of false decisions in conformity assessment of a substance or material with a mass balance constraint (IUPAC Technical Report)

A2 Expt 14.4 (8) Analysis of Iron Tablets, PDF, Iron

Enhanced phytoremediation of Metal(loid)s via spiked ZVI nanoparticles: An urban clean-up strategy with ornamental plants - ScienceDirect

PDF) Analytical-Chemistry Laura G Anzaldo
de
por adulto (o preço varia de acordo com o tamanho do grupo)