hydrogen orbital wavefunction
Por um escritor misterioso
Descrição
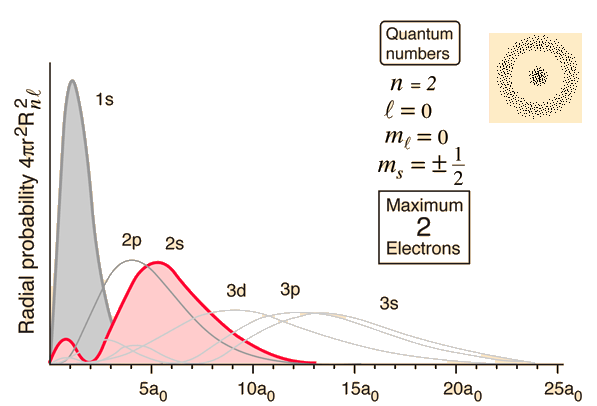
Hydrogen Radial Probabilities

88.18 -- Electron cloud models
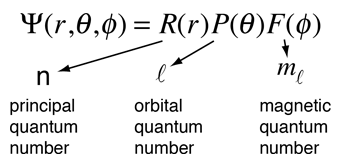
Hydrogen Schrodinger Equation

First normalized wave functions of the hydrogen atom. This is an


quantum mechanics - How do we decide whether an electron orbital has a non-zero or zero probability of lying inside the nucleus of an hydrogen atom? - Physics Stack Exchange
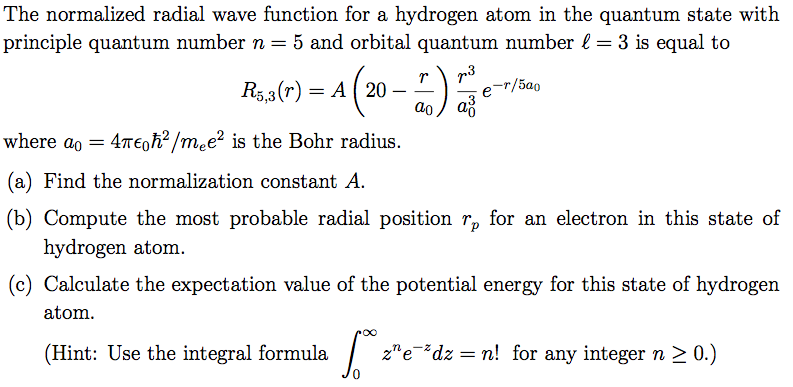
Solved The normalized radial wave function for a hydrogen
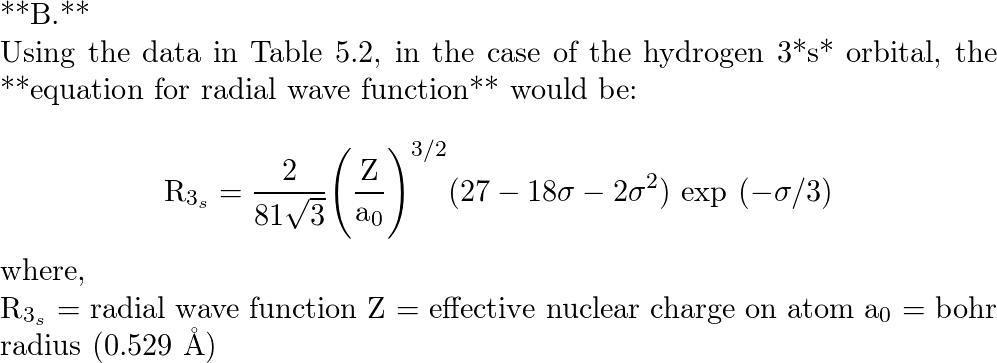
a) Use the radial wave function for the 3p orbital of a hyd
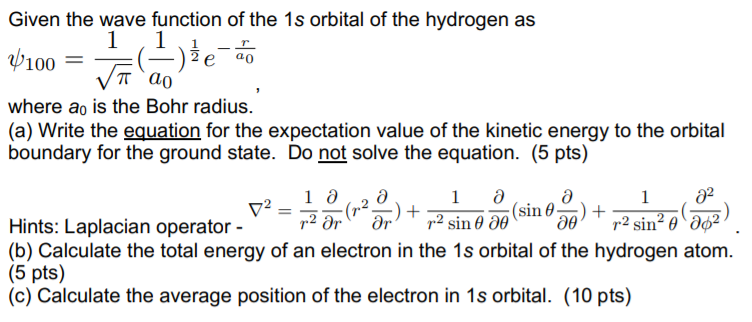
Solved Given the wave function of the 1s orbital of the

Radial Wave Function and Angular Wave Functions, PDF, Atomic Orbital

11.10: The Schrödinger Wave Equation for the Hydrogen Atom - Chemistry LibreTexts

Hydrogen Atom Wave Function Electron Orbital (4,3,0) Poster for Sale by cbeuw

The Schrodinger wave equation hydrogen atom is: Psi_{2s}= dfrac{1}{4 sqrt{2 pi}} left( dfrac{1}{a_{0}} right)^{3/2} left( 2- dfrac{r_{0}}{a_{0}} right) e^{-dfrac{r_{0}}{a_{0}}}, where a_{0} is Bohr's radius. If the radial node is 2s be r_{0}
de
por adulto (o preço varia de acordo com o tamanho do grupo)