What Does the IRB Review?, Research
Por um escritor misterioso
Descrição
Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
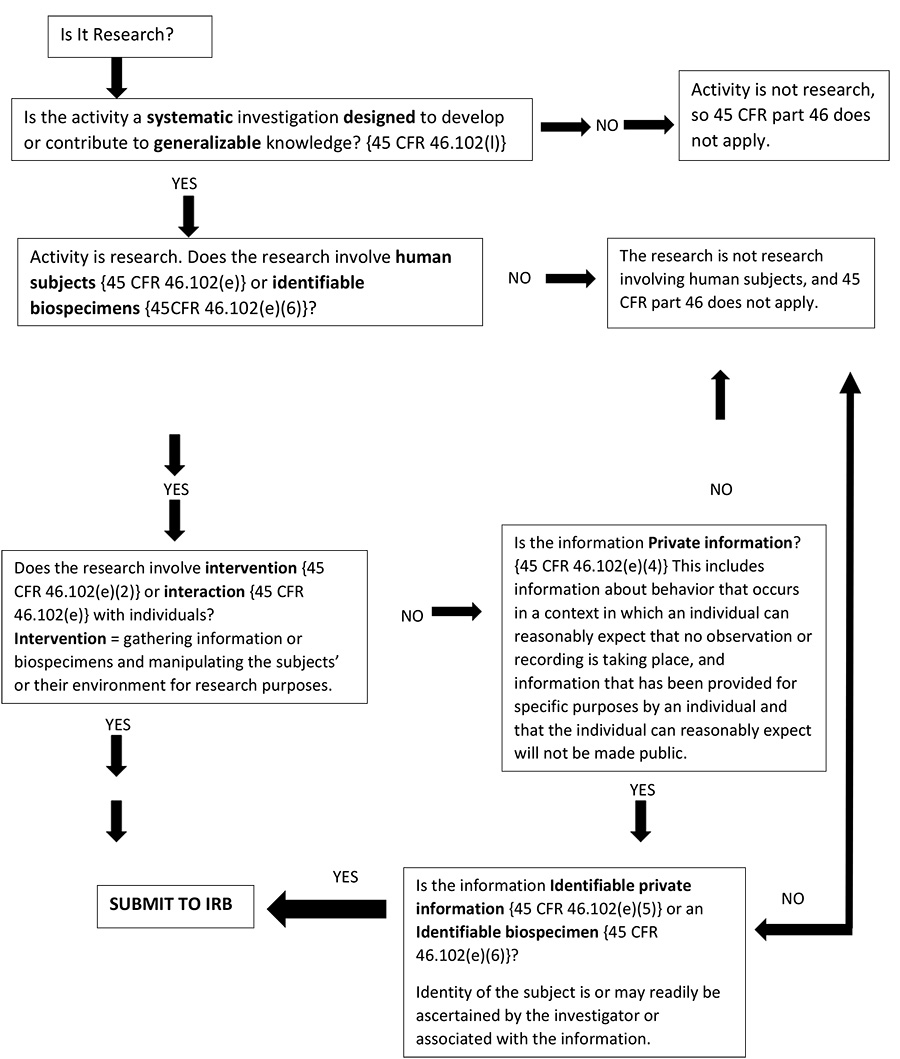
Does Your Research Project Require IRB Review?
Activities Requiring IRB Submission

Human Subjects Research
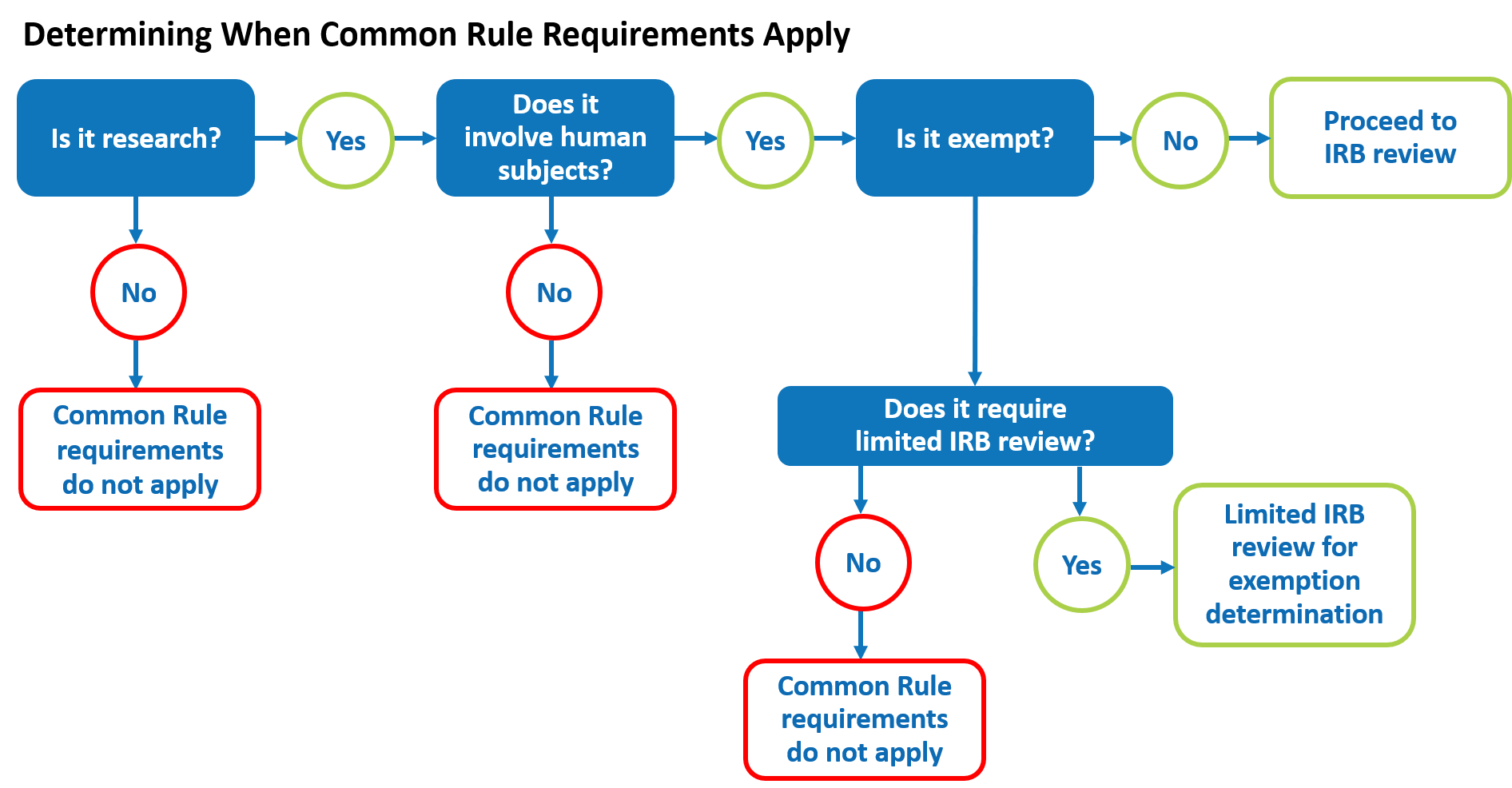
Lesson 2: What is Human Subjects Research?
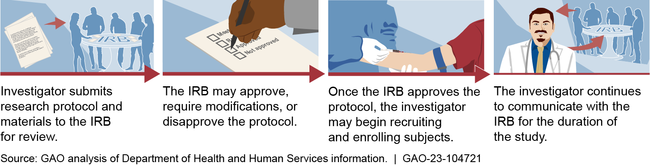
Institutional Review Boards: Actions Needed to Improve Federal Oversight and Examine Effectiveness
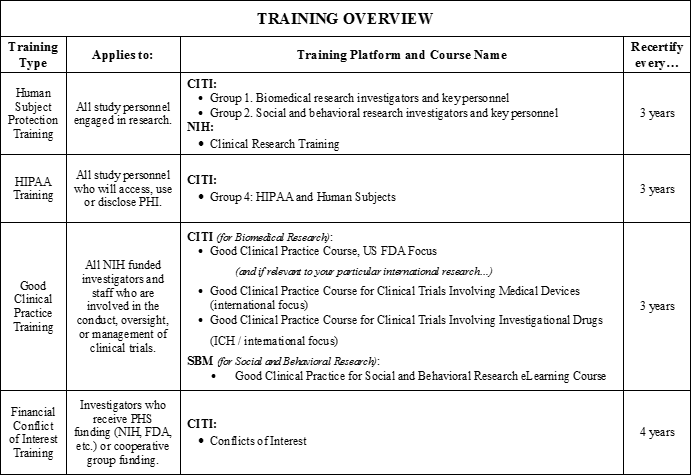
IRB 101, Office of Research Oversight/Regulatory Affairs

Activities Requiring IRB Review - Office of Research Support and Compliance

Human Subject Participation - Office of Research Integrity
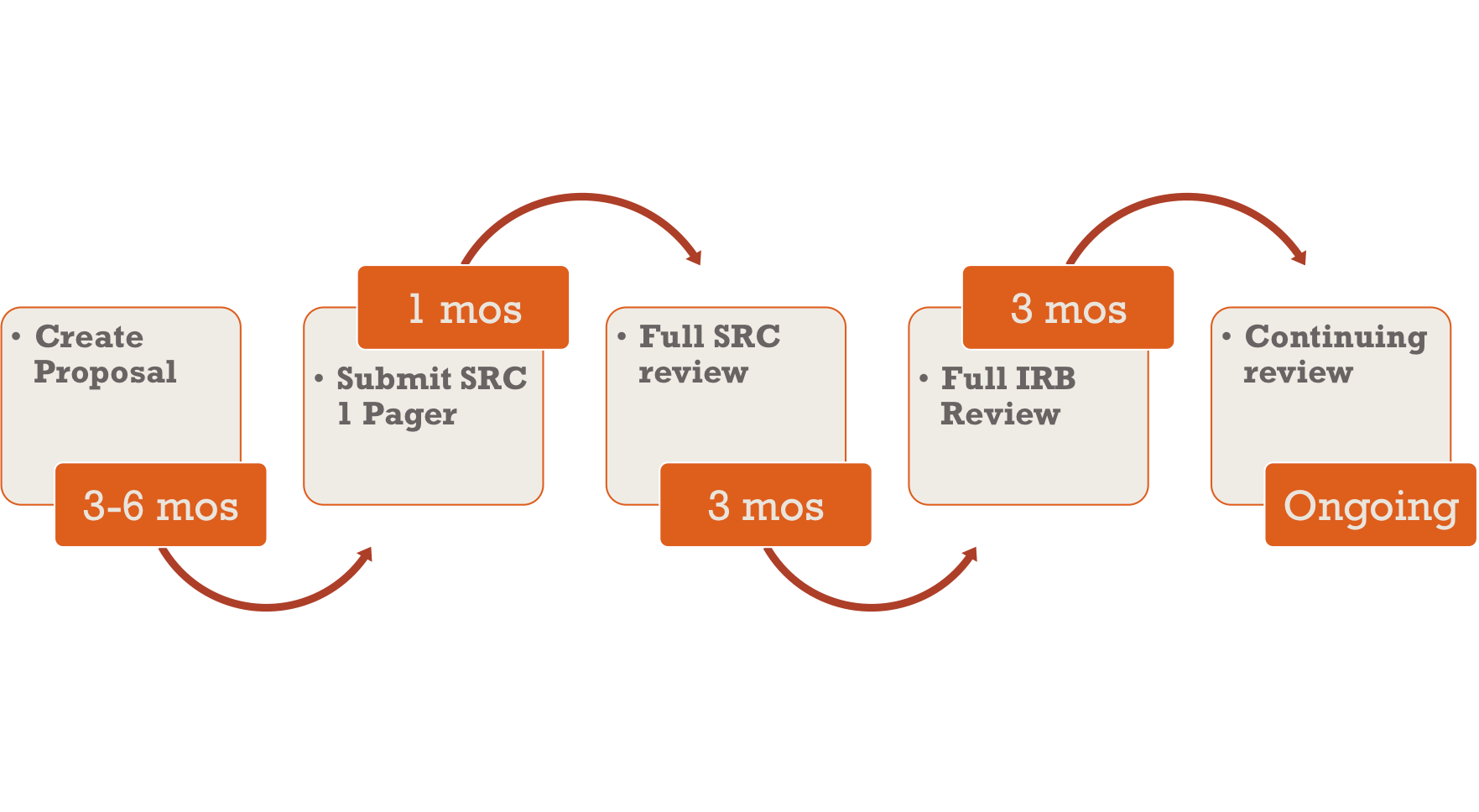
Scientific Review and IRB Submissions - National University of Natural Medicine
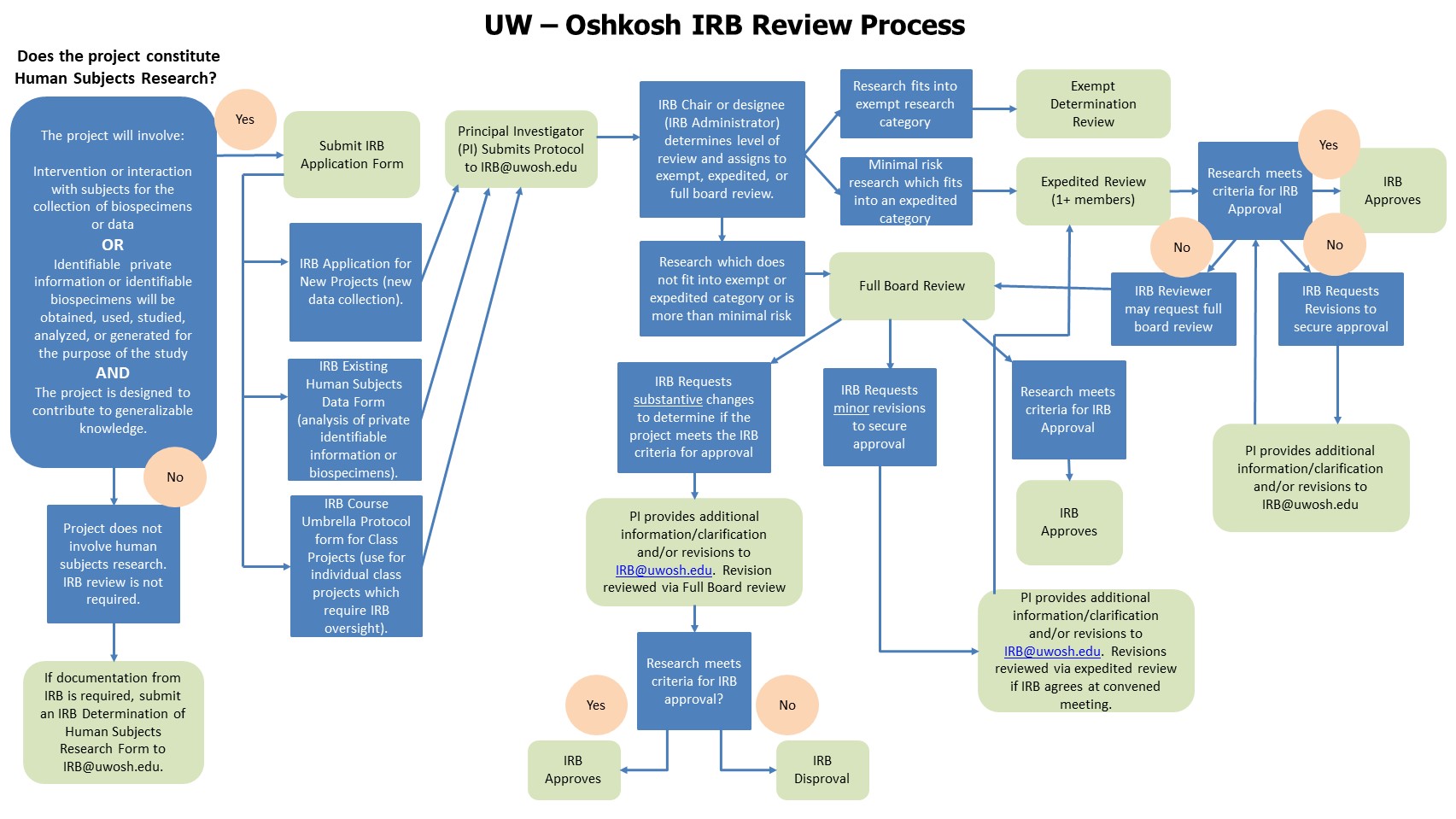
IRB Process - Office of Sponsored Programs University of Wisconsin Oshkosh
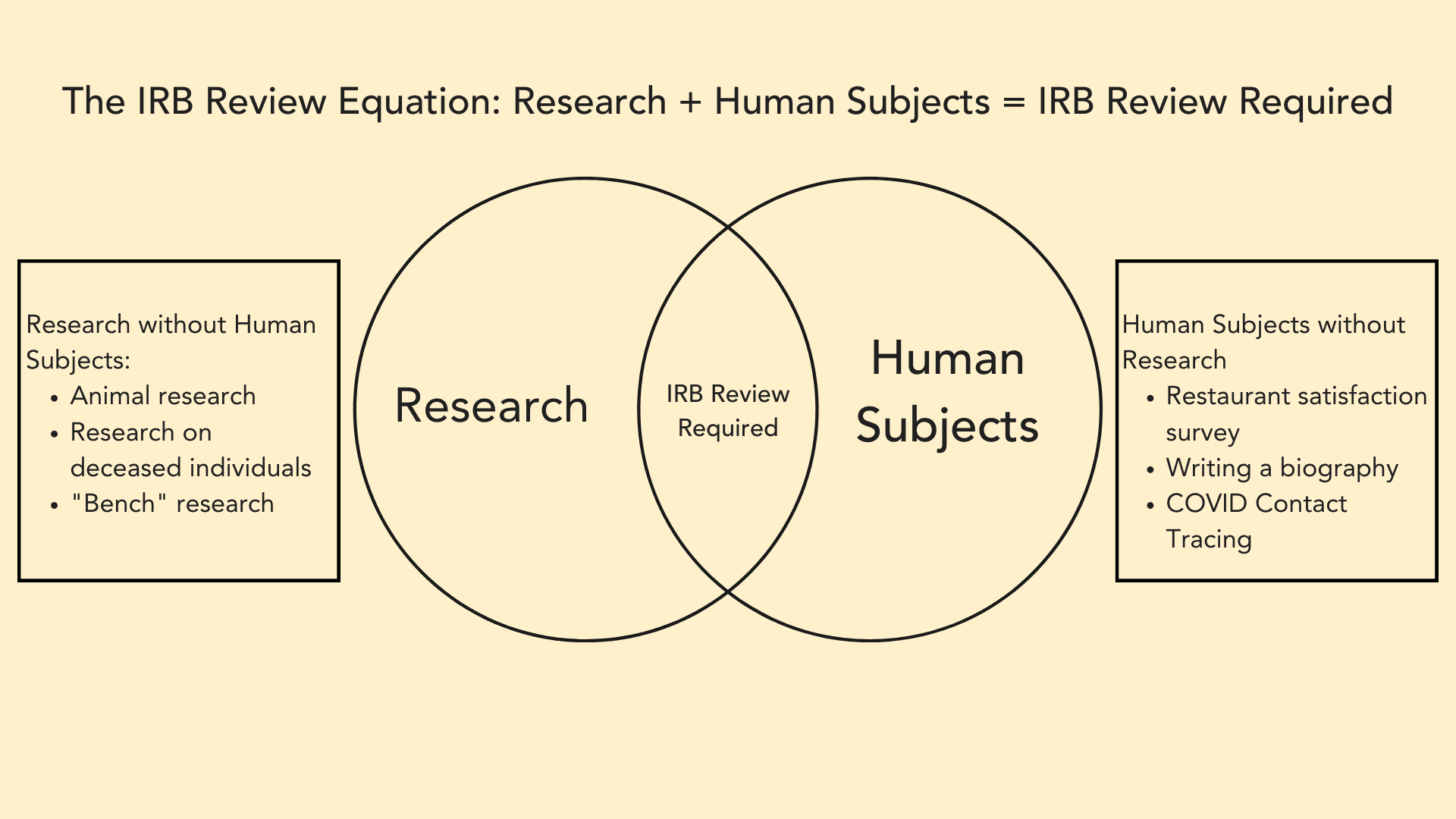
Deep Dives: What is the Difference Between “Exempt” Human Subjects Research, and Projects that are Not Human Subjects Research (NHSR)? – VCU Human Research Protection Program (HRPP) Blog
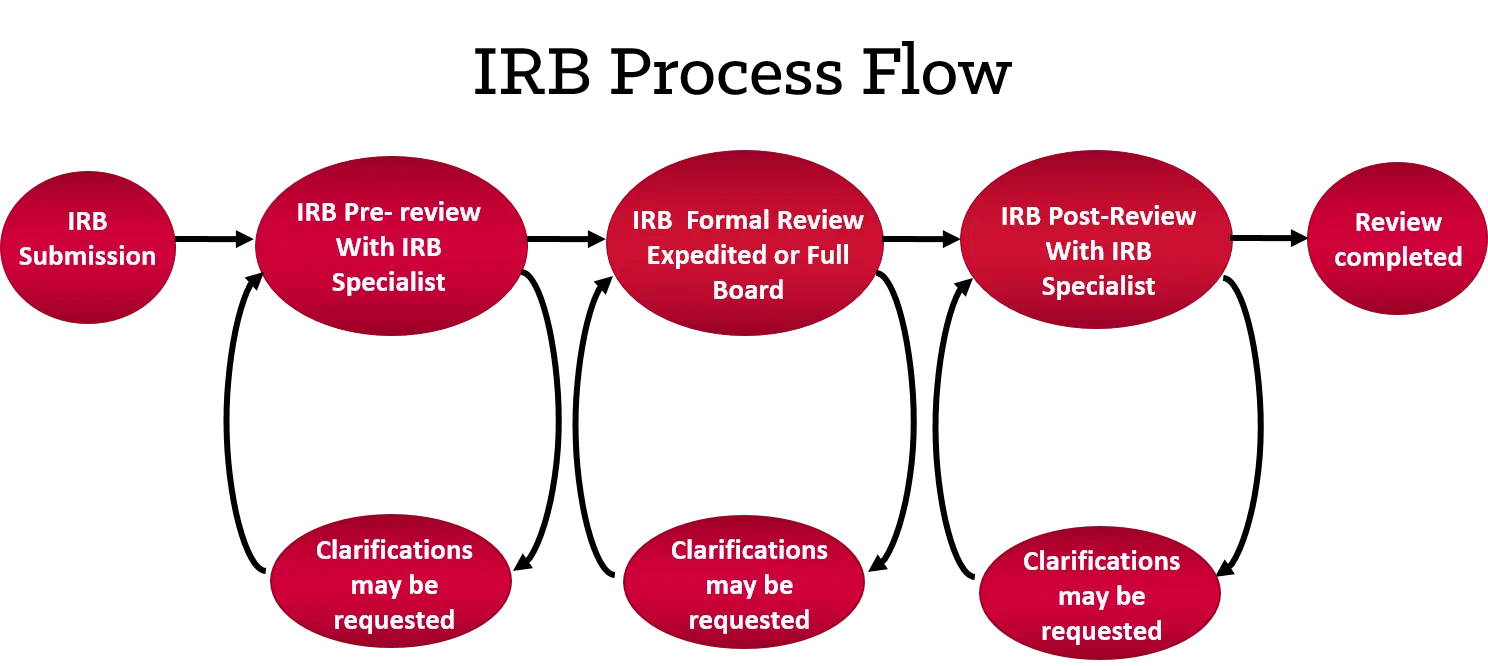
Institutional Review Board, Human Research Protection Program, University Hospitals, Cleveland, OH
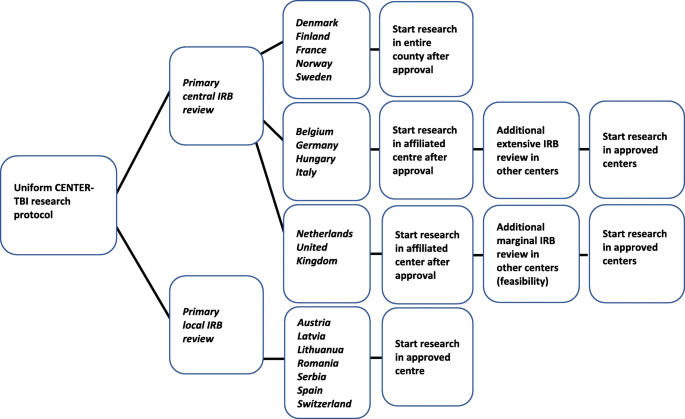
How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study, BMC Medical Ethics

PDF) The reporting Of IRB review in journal articles presenting HIV research conducted in the developing world

What is an IRB?
de
por adulto (o preço varia de acordo com o tamanho do grupo)







