In Vitro vs In Vivo Preclinical Studies
Por um escritor misterioso
Descrição
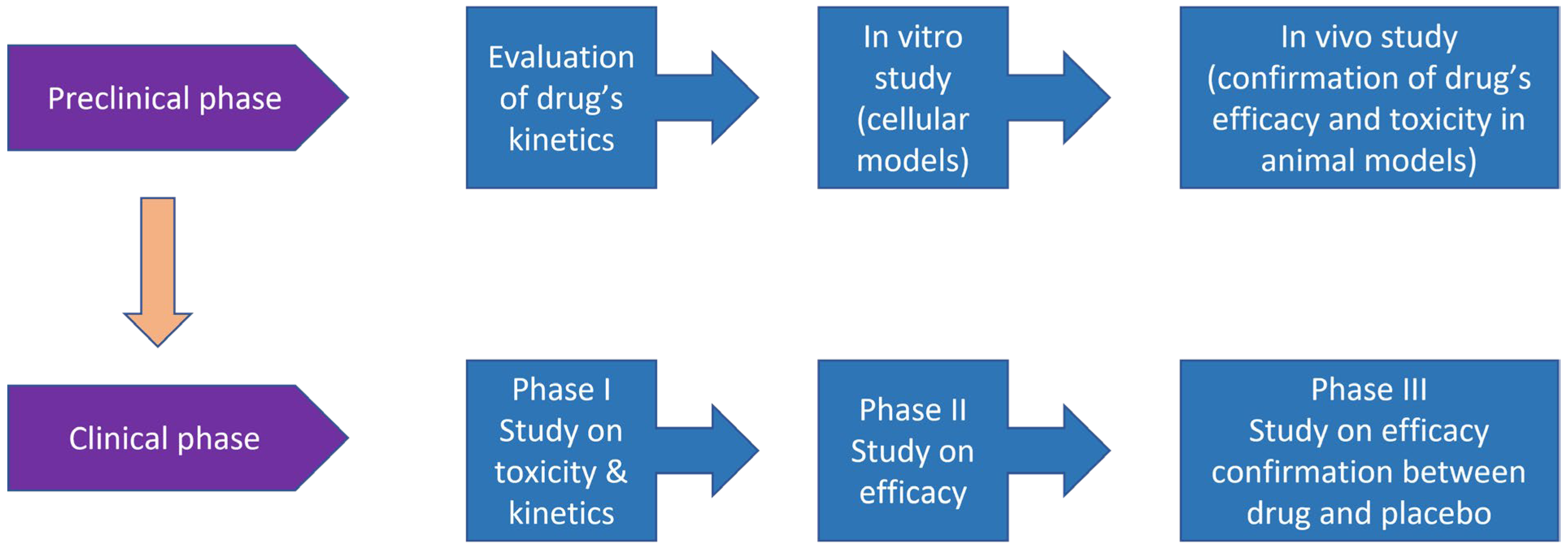
Before a drug candidate can be tested in humans, its safety and efficacy must be explored in in vitro or in vivo preclinical studies.

Limitations of Animal Studies for Predicting Toxicity in Clinical
In Vivo vs In Vitro: Differences in Early Drug Discovery

Experimental Program Design Considerations for Optimized Use of

Biomarker Discovery and Validation Using a Combination of In Vitro

Perspectives on the translation of in-vitro studies to precision
Life, Free Full-Text
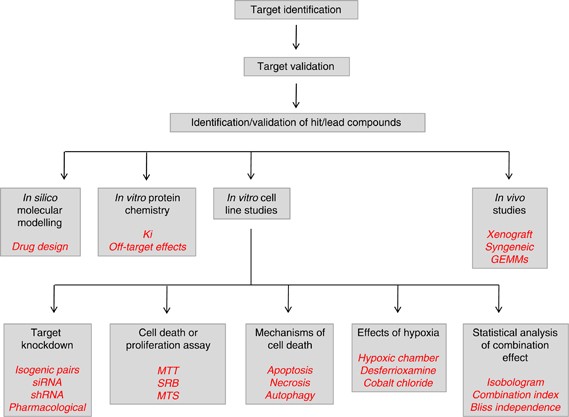
Guidelines for preclinical and early phase clinical assessment of

In Vitro vs. In Vivo Preclinical Drug Testing

Our Guide To Success In Preclinical Data Analysis
Large scale meta-analysis of preclinical toxicity data for target

Ex Vivo vs In Vitro: Understand the Difference
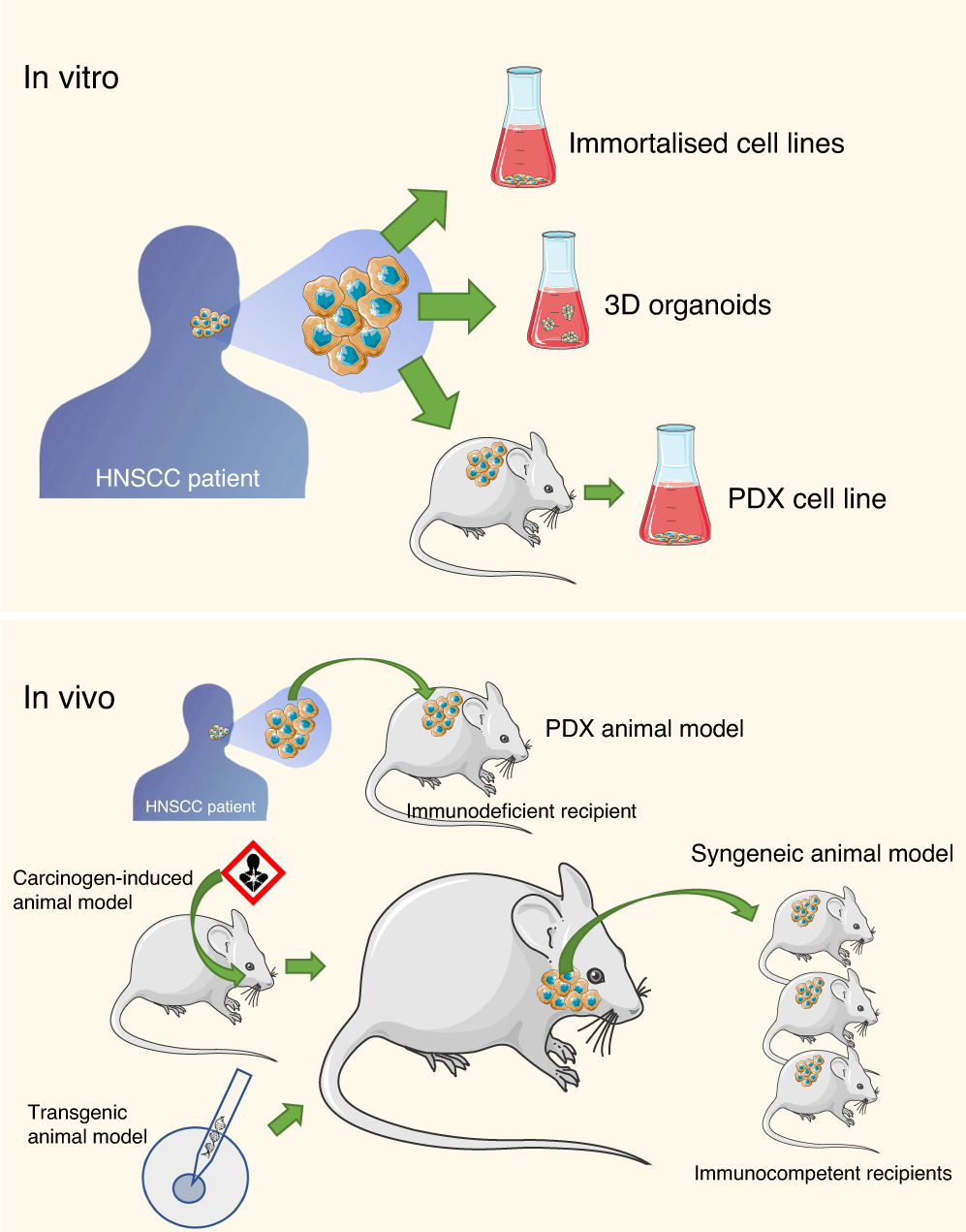
Preclinical models in head and neck squamous cell carcinoma

In vivo vs. in vitro: What is the difference?

The potential of organ-on-a-chip. The conventional drug
de
por adulto (o preço varia de acordo com o tamanho do grupo)