Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Descrição
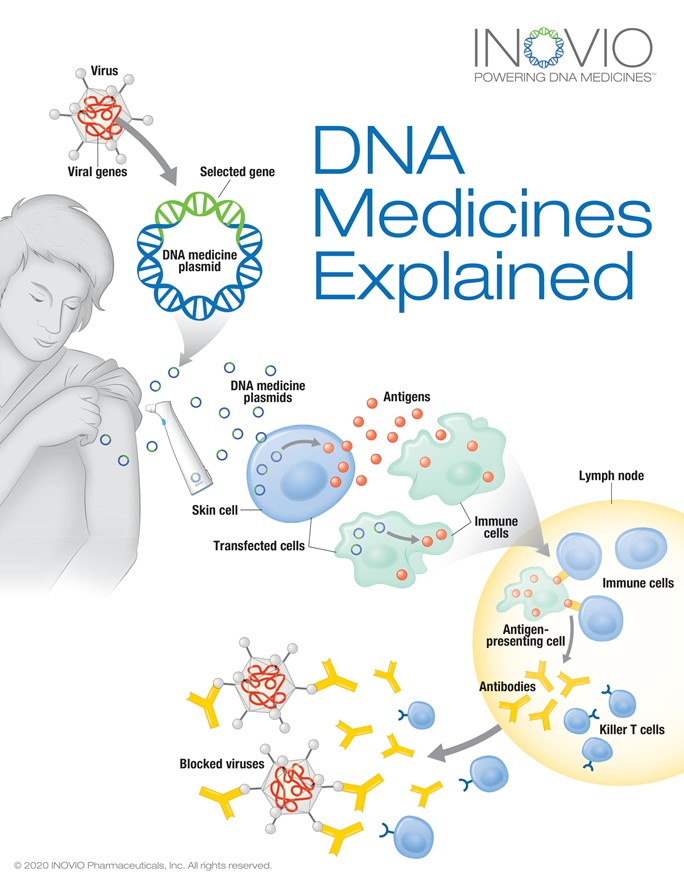
ino-20201231

Immunology and Technology of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccines

Inovio commences Phase I trial of DNA vaccine for Covid-19

An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations - Bulent Kantarcioglu, Omer Iqbal, Joseph Lewis, Charles A. Carter, Meharvan Singh, Fabio Lievano
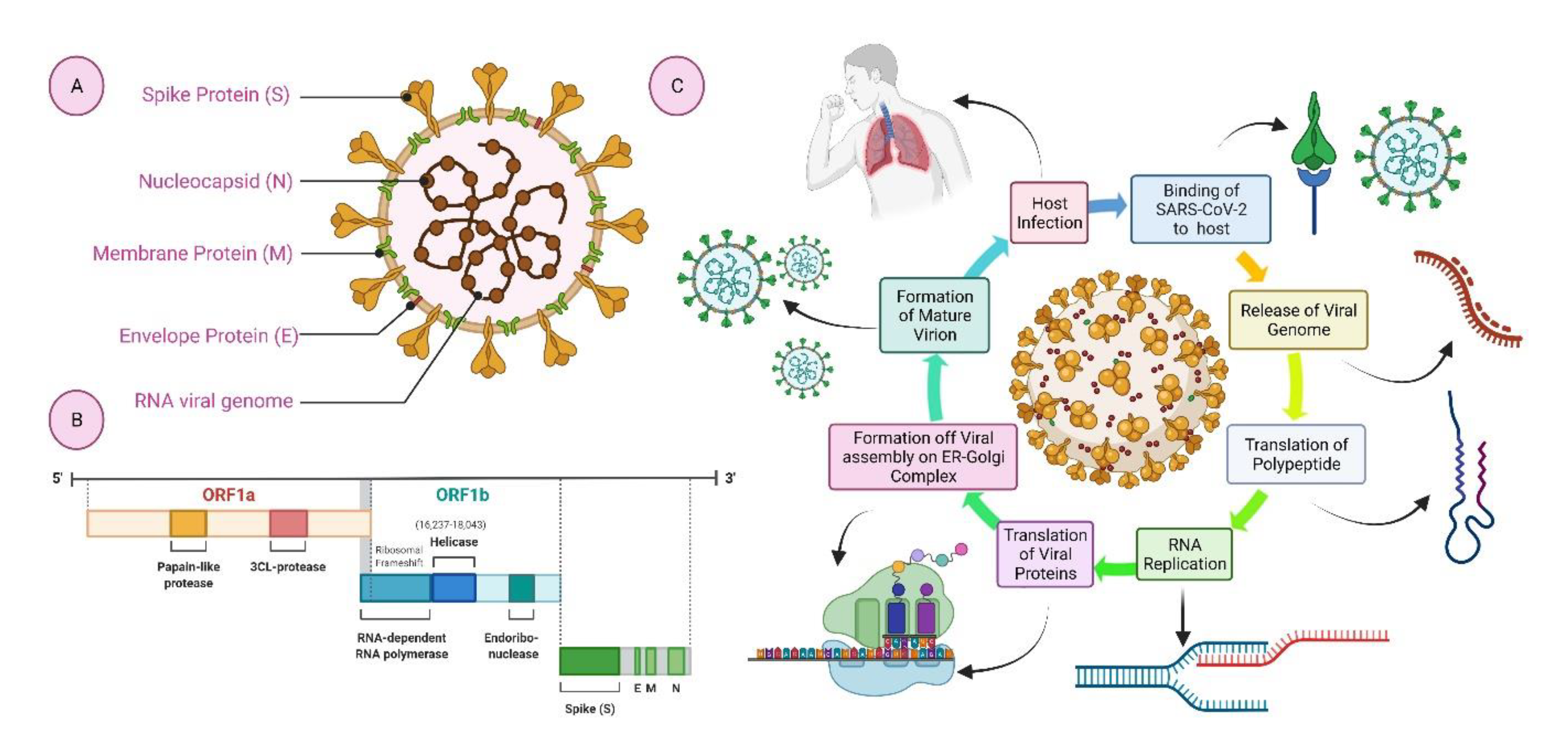
Biologics, Free Full-Text
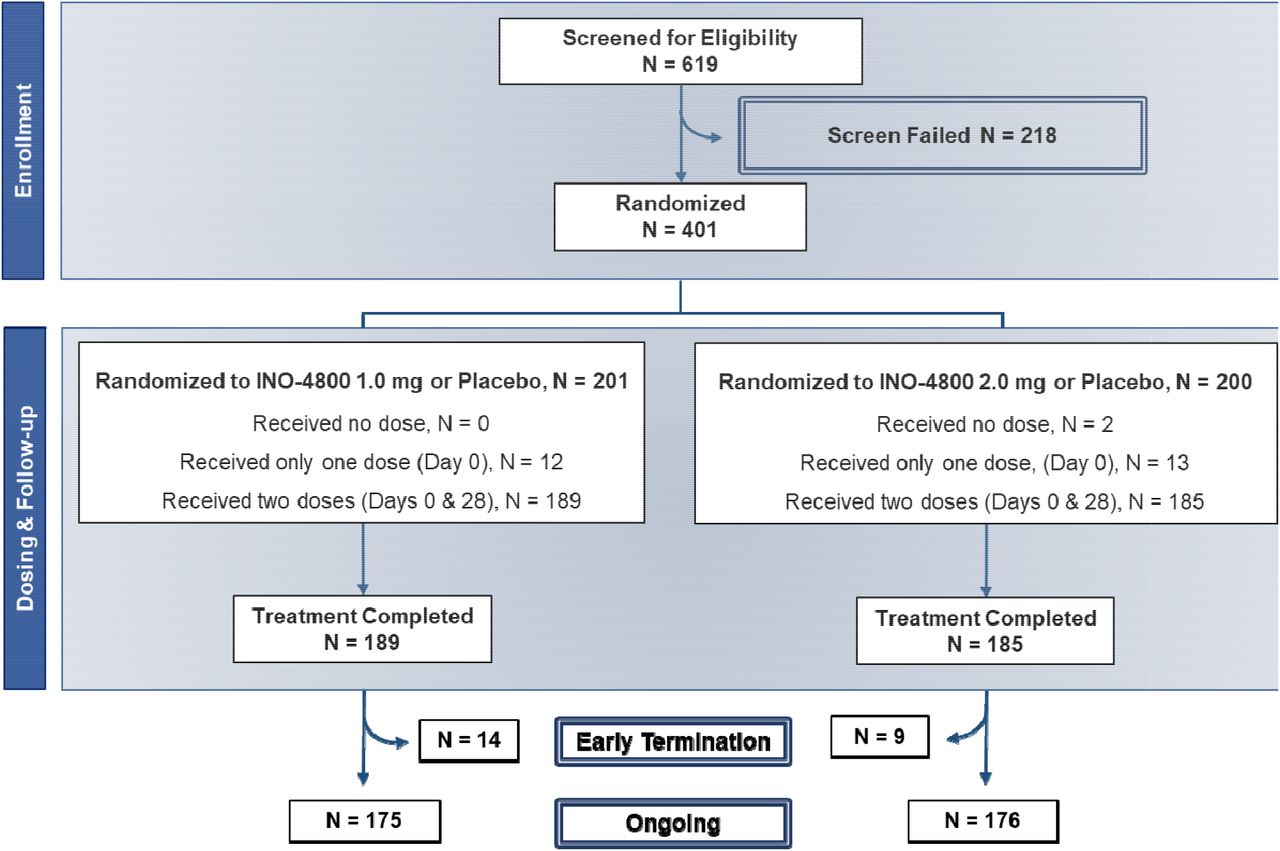
Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure
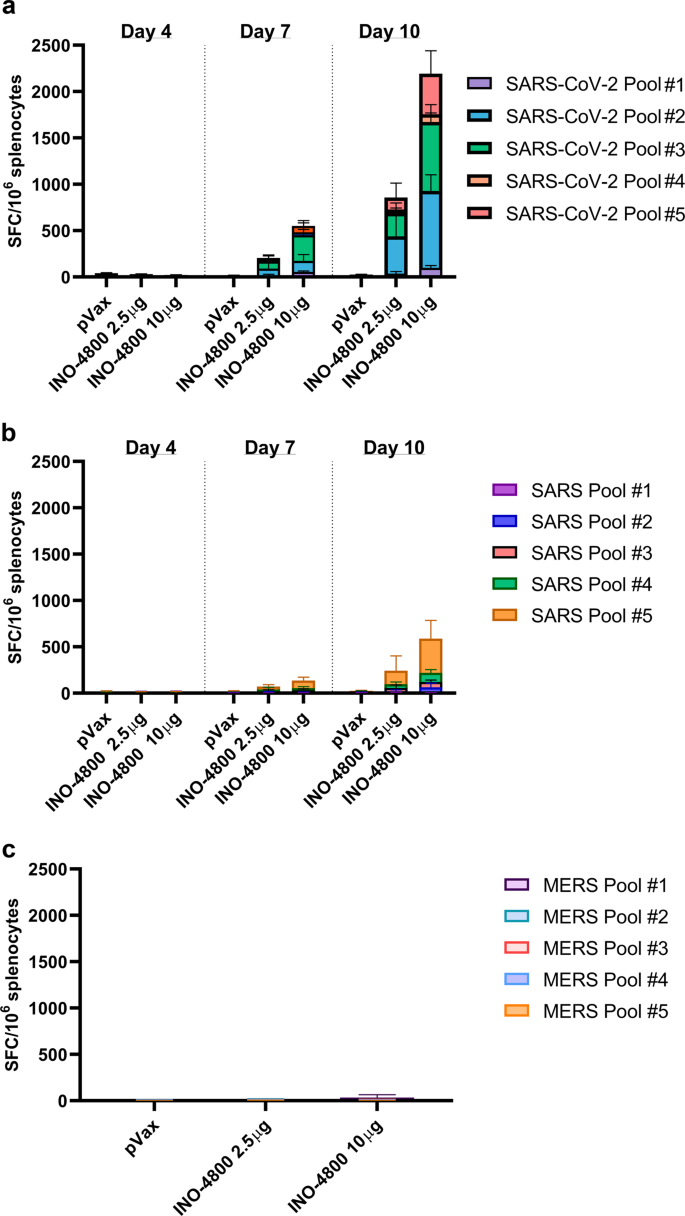
Immunogenicity of a DNA vaccine candidate for COVID-19
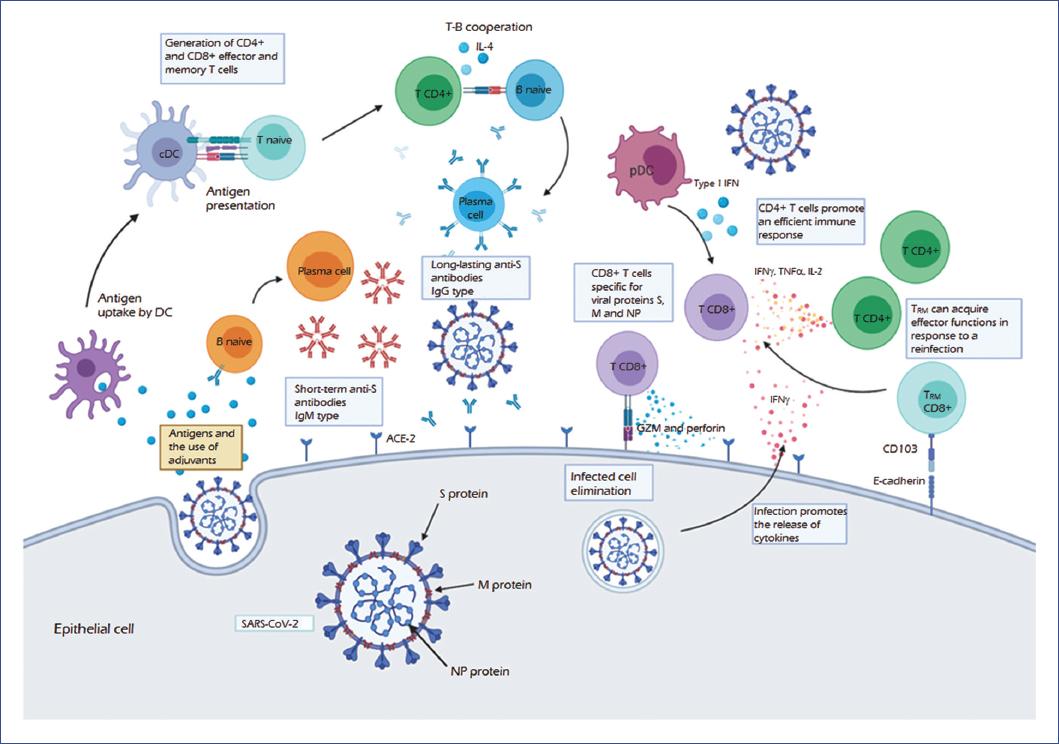
SARS-CoV-2: previous coronaviruses, immune response, and development of vaccines
DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice

Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India - The Lancet
de
por adulto (o preço varia de acordo com o tamanho do grupo)




:max_bytes(150000):strip_icc()/LG-QNED_LifeStyleShoot_Gaming-ead8c5d23c054277ab55bb7d53cb7ef9.jpeg)

