Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Strategies to Control or Mimic Growth Factor Activity for Bone

AusperBio Announces FDA Clearance of IND Application of AHB-137 in
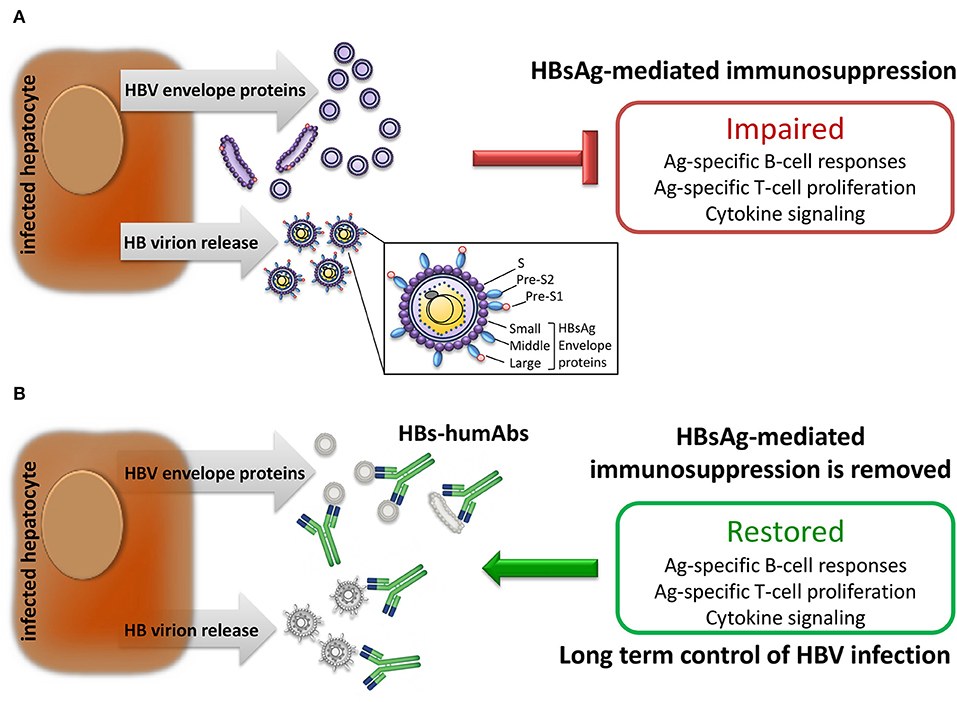
Frontiers Human Monoclonal Antibodies as Adjuvant Treatment of

Hepatitis B Immune Globulin (Human) HyperHEP B®

LinkedIn Landon Loving 페이지: Biotech pipeline hosts 163

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of
Biotech Fierce Biotech
Hepatitis B drug developers chart slow progress, just like in hep C
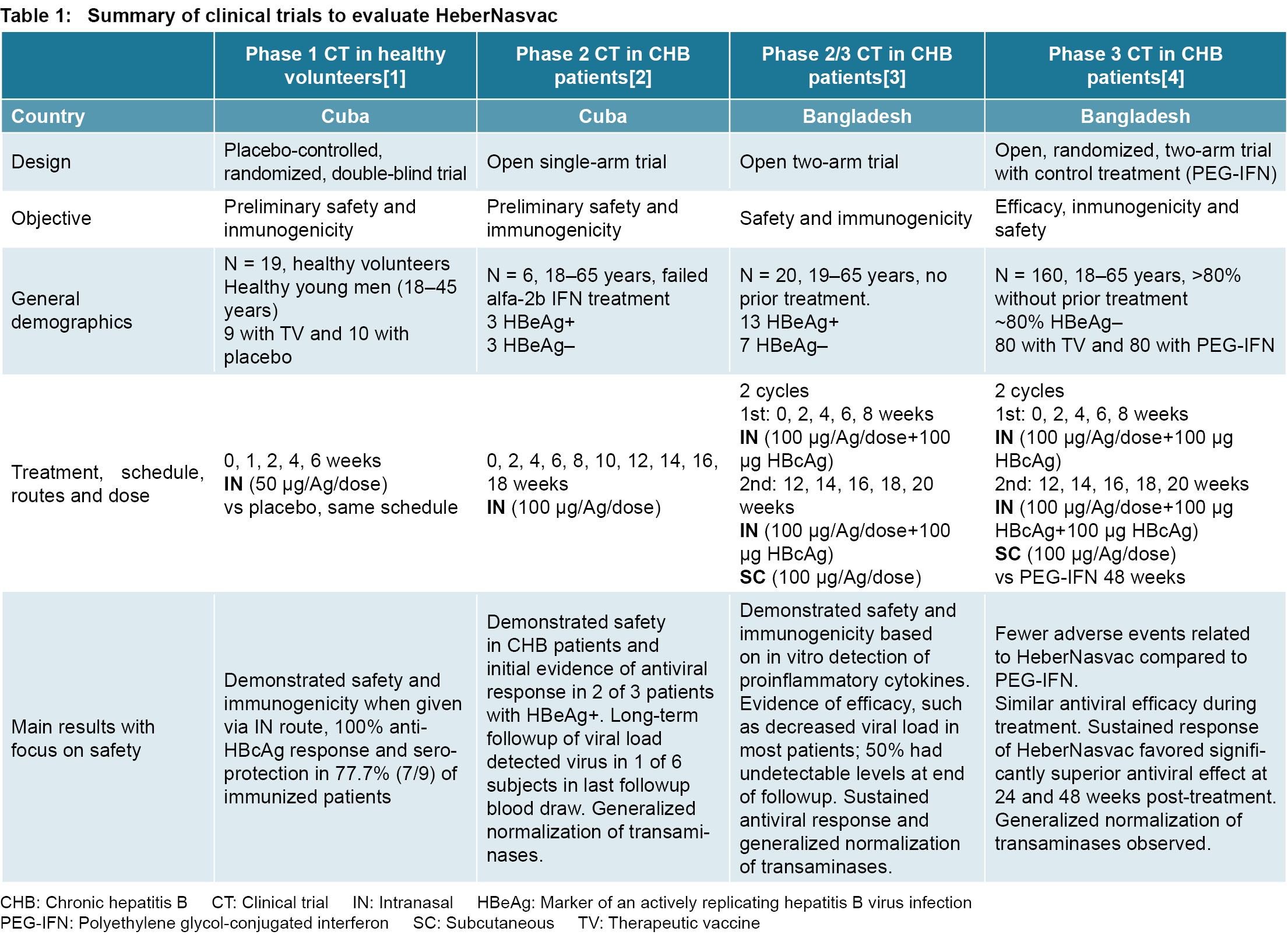
MEDICC Review Cuban Prophylactic and Therapeutic Vaccines for

Microfluidic Formulation of Topological Hydrogels for Microtissue
Clinical Hold on Antios' HBV Therapy Ends Deal with Assembly
Biotechs jockey for gene therapy lead with hemophilia data

Therapeutic vaccination for treatment of chronic hepatitis B

Annalee Armstrong - Journalist Profile - Intelligent Relations
de
por adulto (o preço varia de acordo com o tamanho do grupo)







