Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Descrição

Econsent Solutions: A Better Way to Process Informed Consent in
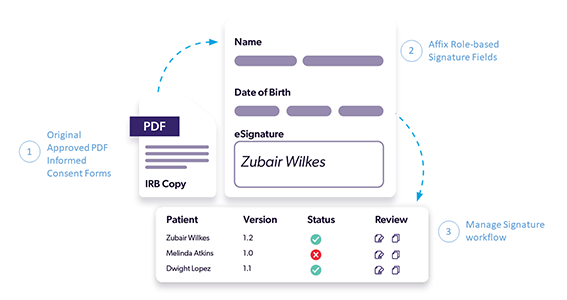
Scaling up eConsent
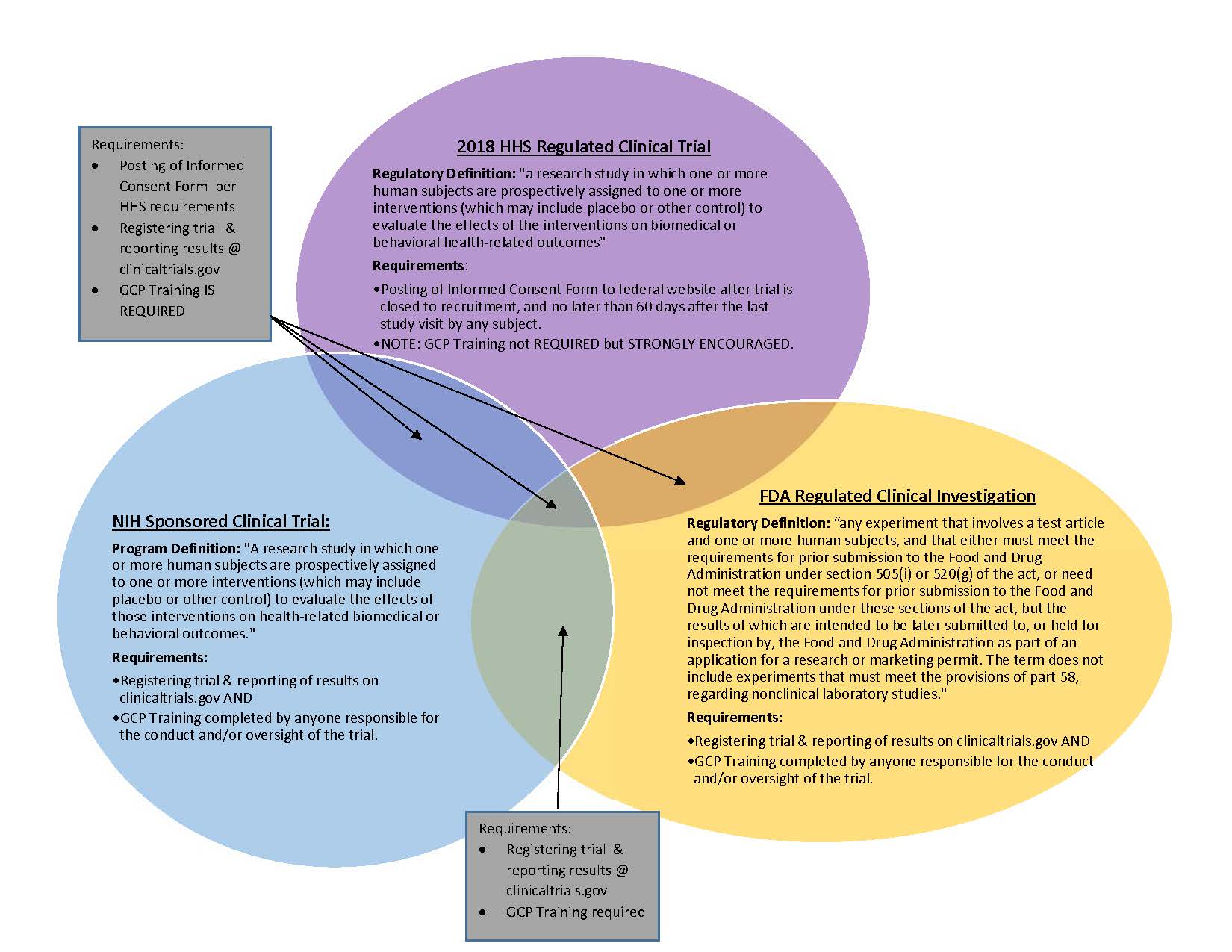
Clinical Trials Research and Innovation

Paving the way to a more effective informed consent process
How to Avoid the Top 5 Clinical Trial FDA Inspection Failures

eConsent Forms Benefits for Clinical Trials

8 Clinical Trial Trends in 2023
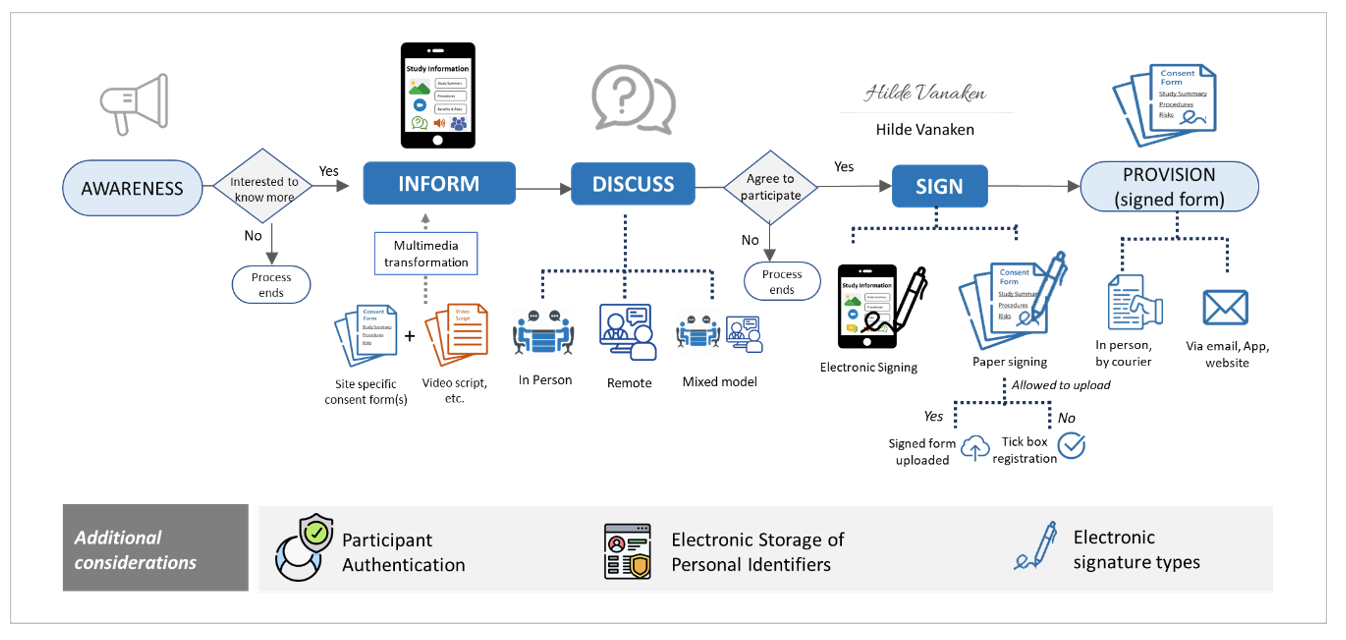
eConsent—The First Step to Enable Clinical Trial Access to Anyone
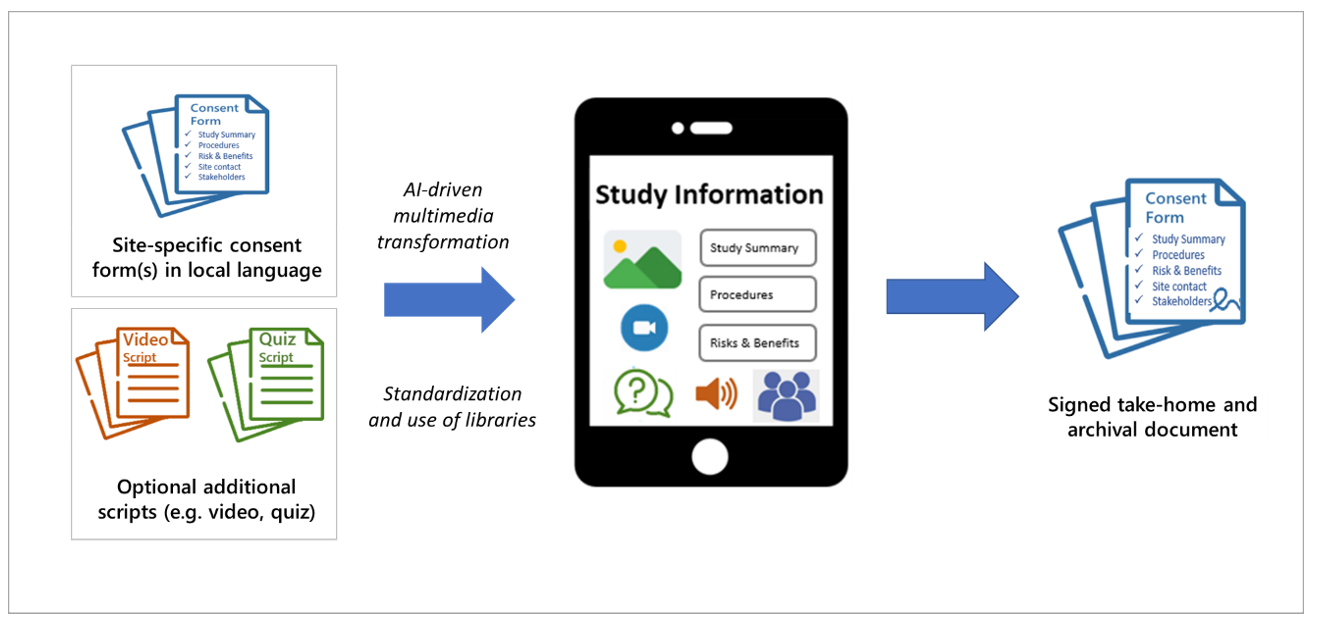
eConsent—The First Step to Enable Clinical Trial Access to Anyone

The informed consent form navigator: a tool for producing readable
Top 4 eConsent Questions in Clinical Research: Forms & More
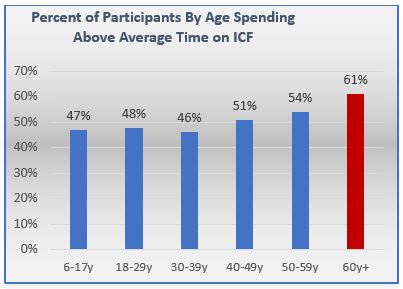
eConsent In Clinical Trials Insights For Implementation During

Leveraging Mobile Technology to Improve Efficiency of the Consent
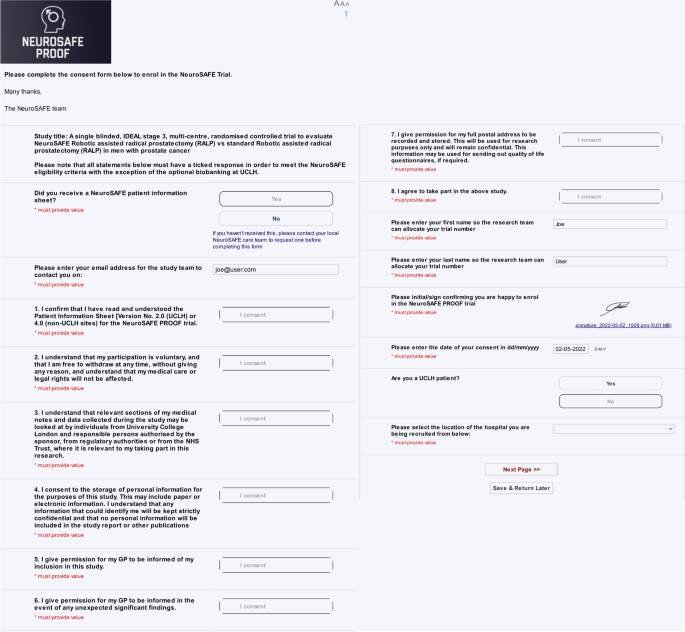
E-Consent—a guide to maintain recruitment in clinical trials
de
por adulto (o preço varia de acordo com o tamanho do grupo)