GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Descrição
GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g

Solved 6.00 g of a certain Compound X, known to be made of
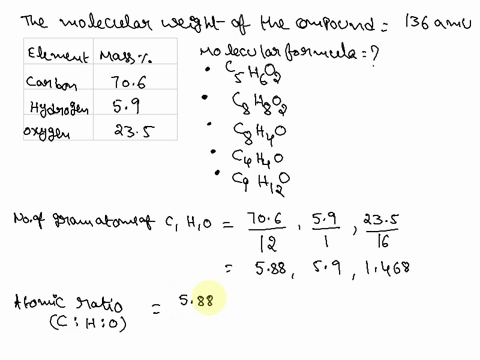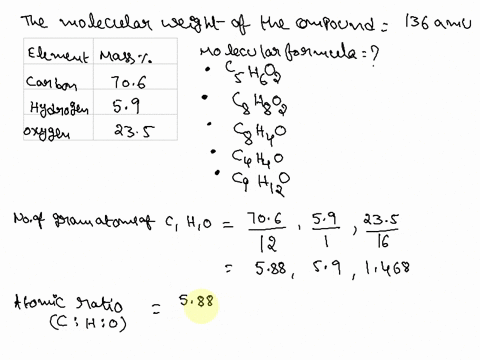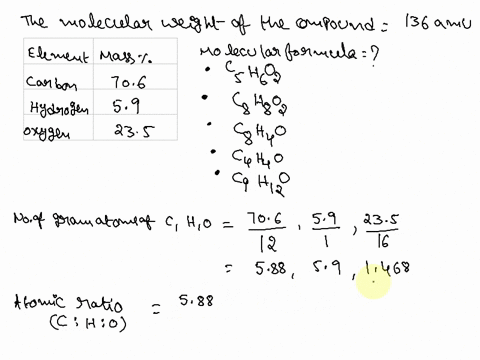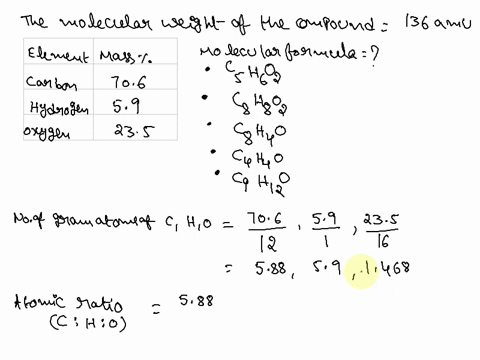
SOLVED: Question 15 (2 points) Determine the molecular formula for a compound that is 46.16% carbon; 5.16% hydrogen; and 48.68% fluorine: The molar mass of the compound is 156.12 grams. CaH4F CsH4F CsHF4 CaHF2
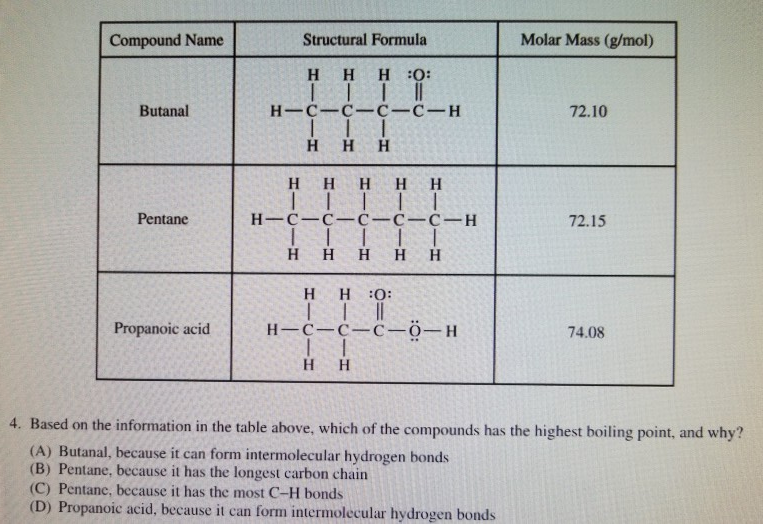
Solved Compound Name Structural Formula Molar Mass (g/mol)

A compound is found to contain 39.99% carbon, 6.727% hydrogen, and 53.28% oxygen by mass. The molar mass for this compound is 90.09 g/mol. What is the molecular formula for this compound?
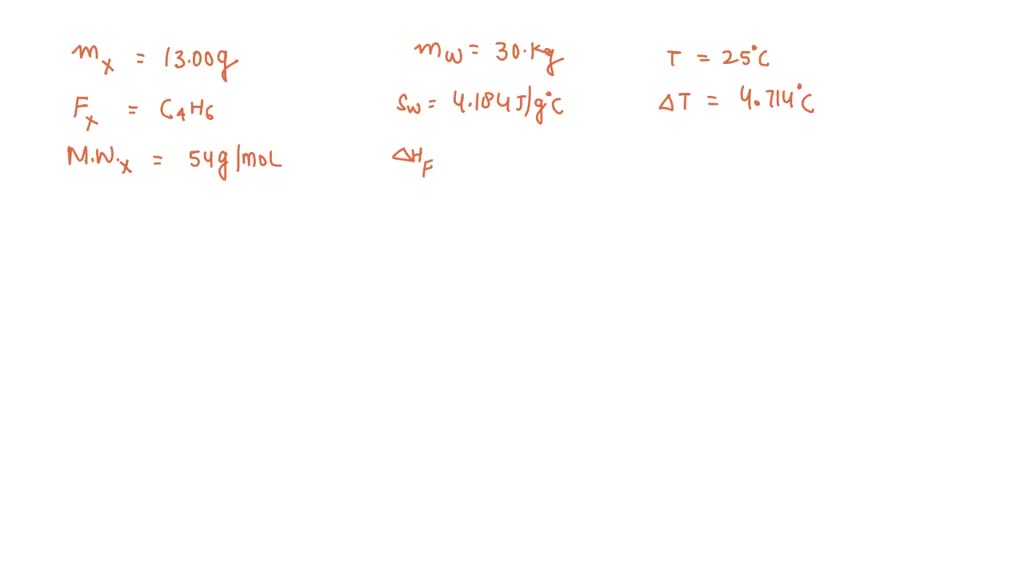
SOLVED: 13.00 g of Compound X with molecular formula C4H6 are burned in constant-pressure calorimeter containing 30.00 kg of water at 25 %C The temperature of the water is observed to rise

4-Heptanone SDF/Mol File - C7H14O - Over 100 million chemical compounds

SOLVED:What is the mass of the molecular ion formed from compounds having each molecular formula: (a) C3H6O; (b) C10H20; (c) C8H8O2; (d) methamphetamine (C10H15N)? How to use the mass of the molecular
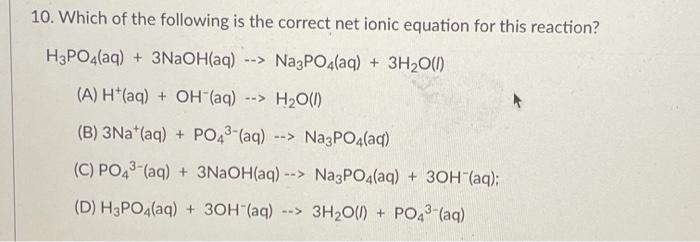
Solved 11. A compound has the composition of 66.6% Carbon
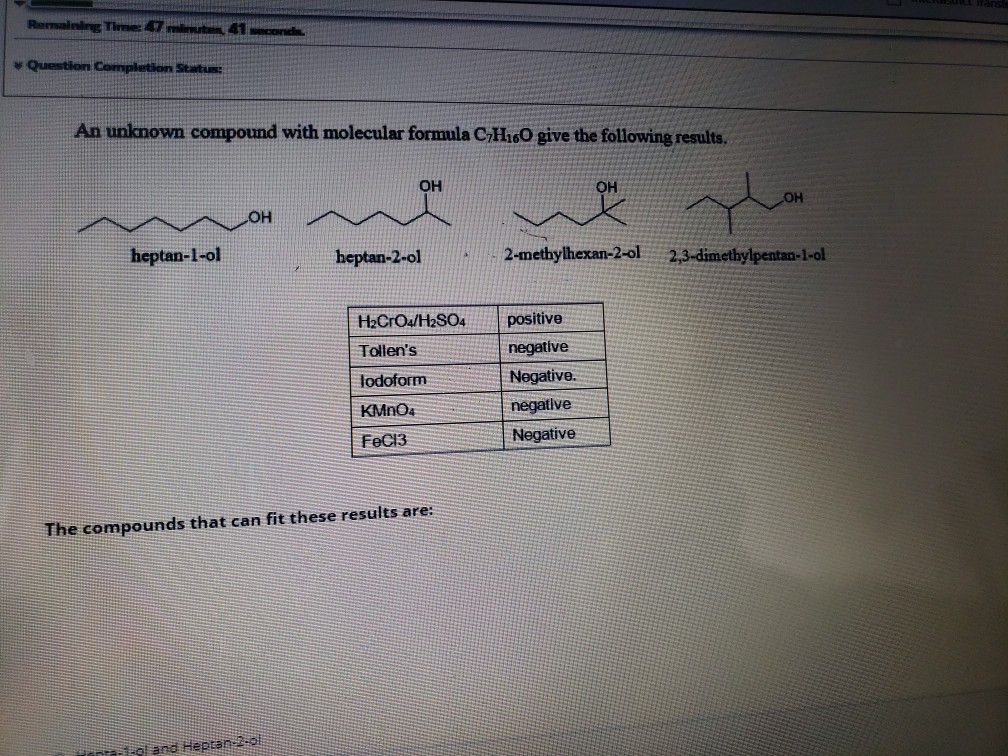
Solved Remaining Time: 41 seconds Question Completion

Anthraquinone contains only carbon, hydrogen, and oxygen. Wh
de
por adulto (o preço varia de acordo com o tamanho do grupo)







